Antibody-drug Conjugates: A Comprehensive Guide
Antibody-drug conjugates (ADCs) are a potent combination of chemotherapy and immunotherapy. The concept was first proposed more than 100 years ago by German scientist Paul Ehrlich. He likens ADCs to "magic bullets" because they can specifically identify their targets (cancer cells) without harming the organism, much like "snipers." In recent years, with the continuous approval and marketing of ADCs, this kind of drug has become one of the hottest technology fields in the current global biotechnology drug research and development. So, why are ADCs so amazing? How do they achieve "precision guidance"? This article collects several FAQs of ADCs, including:
1. What are Antibody-drug Conjugates?
Antibody-drug conjugates (ADCs) are new biological drugs, which combine the high specificity of monoclonal antibody drugs with the high activity of small molecule cytotoxic drugs to improve the targeting of tumor drugs and reduce the toxic and side effects.
2. Antibody-drug Conjugates Structure
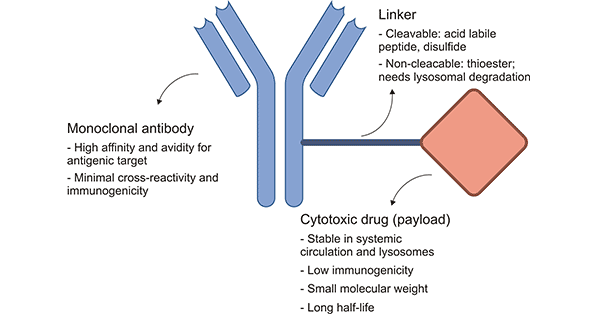
ADCs consist of three main parts: antibodies responsible for selectively recognizing cancer cell surface antigens, payloads responsible for killing cancer cells, and linker used to connect antibodies with Payloads.
Fig.1 the structure of ADCs [1]
3. How do Antibody-drug Conjugates work?
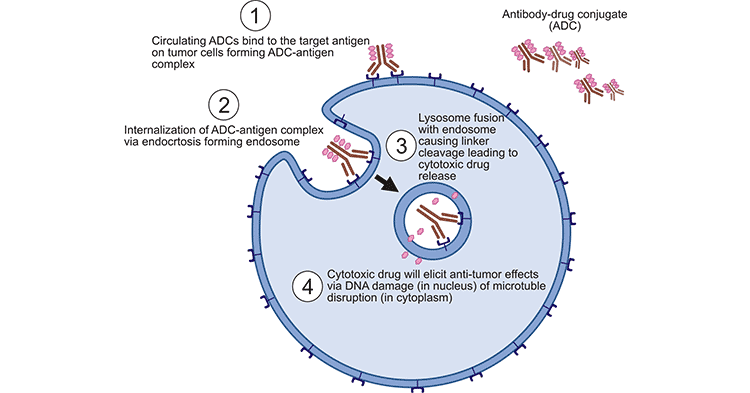
First, the ADCs injected into the body bind to the target cell antigen after many obstacles, and the ADCs-antigen complex formed by it enters the cell through reticulin-mediated endocytosis. After the complex enters the cell, lysosome fusion with endosome causes linker cleavage in the cell, releasing and activating small molecule cytotoxic drugs. After the drug is released into cytoplasm, it can be inserted into DNA or inhibit microtubule polymerization, killing tumor cells and causing apoptosis of target cells. When the target cell dies, the active payloads may also kill the surrounding tumor cells, also known as Bystander effect.
Fig.2 the working mechanism of ADCs [1]
4. Antibody-drug Conjugates Components
4.1 Selection of Targets
The successful development of ADCs depends on the specific binding of antibodies to targets. The ideal targets of ADCs are high expression on the surface of tumor cells, low expression or no expression in normal cells, and minimizing toxicity of on-target and off-tumor. In addition to specificity and sufficient expression, the best target should also cause an efficient internalization effect.
Target antigens in approved ADCs and selected
ADCs in late-stage clinical development
Target antigens regulated from
driver oncogenes
Target antigens in the tumor
vasculature and stroma
Fig.3 Target for ADCs in development and in the clinic and developed
4.2 Selection of Antibodies
Antibodies should have high antigen affinity and long circulating half-life, so that they can specifically enrich cytotoxins in tumor sites. Considering the half-life, structural stability, Fc fragment immune function and coupling convenience of different IgG types antibodies, ADCs mostly choose IgG1 but seldom use IgG4. According to the immunogenicity of antibodies, they can be divided into fully humanized antibodies, humanized antibodies and chimeric antibodies. In order to reduce immune response, completely humanized or humanized antibodies are more selected.
4.3 Selection of Payloads
Toxin molecule (Payload) is the key factor for the success of ADCs research and development. Because ADCs have to go through multiple steps from entering human body to finally releasing cytotoxic agents, considering the efficiency of each step, payloads should have high anti-tumor activity, so toxic molecules with nanomolar level (IC50 value is 0.01-0.1 nM) are the appropriate payloads. In addition, payloads must have suitable functional groups that can be coupled, strong cytotoxicity, proper hydrophilic-hydrophobic balance, and high stability.
4.4 Selection of Linker
Linker, as the bridge of ADCs, connects with antibodies through cleavable connectors or non-cleavable connectors. Linker needs exquisite design, which not only needs stability to prevent chain breaking in the physiological state but also has the characteristics of high release efficiency in specific parts.
5. Advantages of Antibody-drug Conjugates
Compared with traditional completely or partially humanized antibodies or antibody fragments, ADCs have a higher theoretical curative effect because they can release highly active cytotoxins in tumor tissues. On the other hand, ADCs have a higher tolerance and lower side effects than fusion protein. They can accurately identify targets and not affect normal cells, which greatly improves the efficacy and reduces the toxic and side effects, so they have attracted the attention of people in the field of pharmaceutical research and development.
6. Do Antibody-drug Conjugates have any weaknesses?
The structure of ADCs is complex and the design is diversified, which brings difficulties to the production and quality control related to CMC research. At the same time, the superposition of ADCs and complex biological process in vivo also makes non-clinical research and clinical research face multiple challenges. Because the production of ADCs mostly involves highly active cytotoxic drugs, it requires high hardware equipment, process design and personnel training, and requires a large amount of capital investment and technical reserves, so the production threshold is high.
At present, there are three main challenges and opportunities in the development of ADCs:
6.1 Instability of Linker
This instability can lead to premature release of payload into blood, and lead to nonspecific uptake and miss-target toxicity of ADCs.
6.2 Nonspecific Endocytosis
Hydrophobicity will promote the aggregation and nonspecific endocytosis of ADCs, especially ADCs with high DAR (drug-antibody ratio), resulting in miss-target effect. ADCs with high DAR will also be cleared by other cells with nonspecific and strong endocytosis ability. So optimizing DAR is also an important strategy to improve TI (therapeutic index).
6.3 Receptor-mediated Uptake Mechanism
The off-target toxicity of ADC mediated by Fc γ Rs is mainly manifested in blood toxicity. Blood toxicity is the most common off-target dose-limiting toxicity (DLTs) of ADCs containing Auristatin (MAME, MMAF), Calicheamicin and Maytansinoid (DM-1).
7. FDA-approved Antibody-drug Conjugates
Among the ten ADCs approved for marketing, six of them are for the treatment of hematological tumors, and the others are for solid tumors (Table 1). At present, more than eighty ADCs are undergoing active clinical trials, most of which are in Phase I and Phase I/II. More than 80% of clinical trials are studying the safety and effectiveness of ADCs in various solid tumors, while the rest involve hematological malignant tumors. This indicates that following the success of T-DM1 early and the recent approval of sacituzumab govitecan and Loncastuximab tesirine, research on ADCs have gradually turned into the solid tumor in recent years.
Table1 FDA-approved ADCs
| Drug Name |
Trade Name |
Company |
Target |
Payload |
Indications |
Approval year |
| Gemtuzumab ozogamicin |
Mylotarg |
Pfizer/Wyeth |
CD33 |
Calicheamicin |
AML |
2000
2017 |
| Brentuximab vedotin |
Adcetris |
Seattle Genetics, Millennium/Takeda |
CD30 |
MMAE |
cHL、sALCL、PTCL |
2011 |
| Trastuzumab emtansine |
Kadcyla |
Genentech/Roche |
HER2 |
DM1 |
mBC |
2013 |
| Inotuzumab ozogamicin |
Besponsa |
Pfizer/Wyeth |
CD22 |
Calicheamicin |
ALL |
2017 |
| Polatuzumab vedotin |
Polivy |
Genentech/Roche |
CD79b |
MMAE |
r/r DLBCL |
2019 |
| Enfortumab vedotin |
Padcev |
Astellas/Seattle Genetics |
Nectin-4 |
MMAE |
mUC |
2019 |
| Trastuzumab deruxtecan |
Enhertu |
AstraZeneca/ Daiichi Sankyo |
HER2 |
Dxd |
mBC、mGC |
2019 |
| Sacituzumab govitecan |
Trodelvy |
Immunomedics |
TROP2 |
SN-38 |
mTNBC |
2020 |
| Belantamab mafodotin |
Blenrep |
GlaxoSmithKline |
BCMA |
MMAF |
MM |
2020 |
| Loncastuximab tesirine |
Zynlonta |
ADC Therapeutics |
CD19 |
SG3199 |
r/r DLBCL |
2021 |
ADCs have been continuously innovated and optimized since the concept of "biological missile" was put forward and have been greatly improved, making it one of the important means in the field of cancer treatment. As more and more ADCs enter the clinical stages, the industry is gradually shifting from traditional technologies to more innovative technologies to develop this complex product, which includes exploring new tumor antigens, new antibody structures, new payloads, new linkers and advanced coupling methods. With the in-depth study of ADCs, the molecular structure design of ADCs will be more reasonable, the uniformity will be greatly improved, and the stability in vivo will be continuously improved, thus reducing the toxic and side effects, improving the efficacy and activity, expanding the treatment window, and bringing new hope to tumor patients.
CUSABIO ADC Target Antigen Protein Products
Currently, there are 128 ADC targets in development, including 59 with 2 or more pipelines in development. CUSABIO has developed a series of products with different species and tags for a variety of hot targets, which are suitable for immune, antibody screening, SPR, cell activity detection and other experiments. CUSABIO is committed to assisting you in drug development, and all activity test protocols are available for free. In addition, CUSABIO also provides related research antibodies and ELISA kits. If you don't find the targets you desired, you can leave message or chat to us by livechat.
● Partial ADC target Protein Activity Validation Data
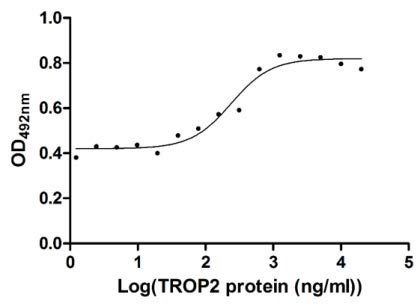
Measured in cell activity assay using U937 cells, the EC50 for this effect is 190.2-298.6 ng/ml.
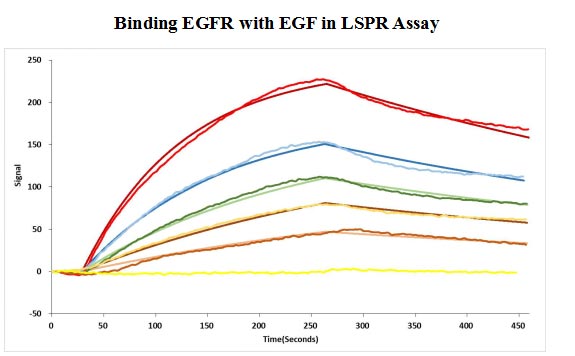
Human EGF protein captured on COOH chip can bind Human EGFR protein, his and Myc tag (CSB-MP007479HU) with an affinity constant of 11.9nM as detected by LSPR Assay.
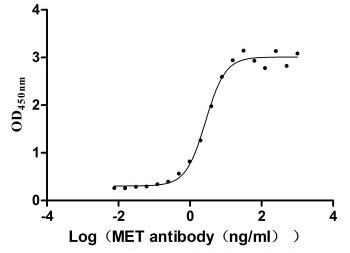
Measured by its binding ability in a functional ELISA. Immobilized MET at 2 μg/ml can bind Anti-MET recombinant antibody, the EC50 is 2.379-3.094 ng/ml.
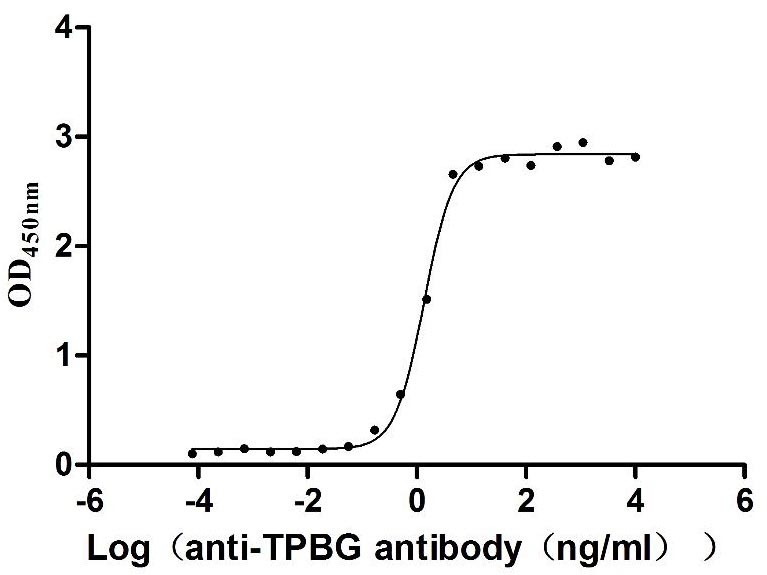
Measured by its binding ability in a functional ELISA. Immobilized Human TPBG at 2 μg/mL can bind Anti-TPBG recombinant antibody (CSB-RA024093MA1HU), the EC50 is 1.230-1.519 ng/mL.
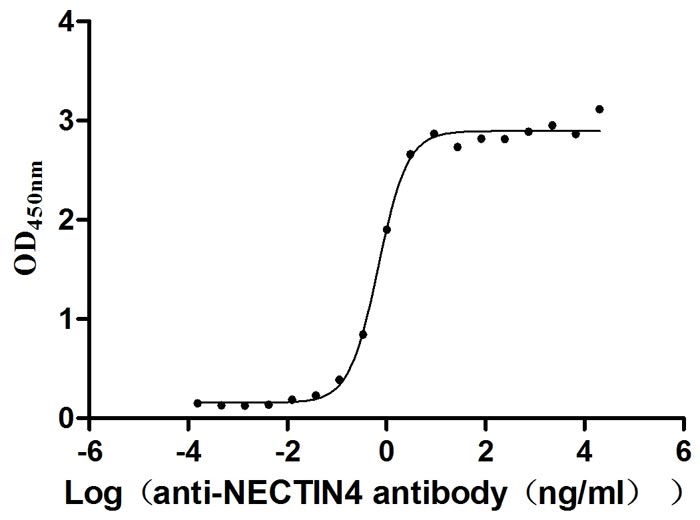
Measured by its binding ability in a functional ELISA. Immobilized NECTIN4 at 2 μg/ml can bind anti-NECTIN4 antibody (CSB-RA822274A0HU) (enfortumab vedotin-like), the EC50 is 0.6029-0.7837 ng/mL.
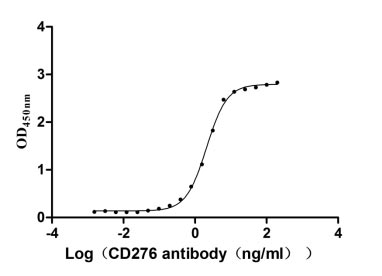
Measured by its binding ability in a functional ELISA. Immobilized CD276 at 2 μg/ml can bind Anti-CD276 rabbit monoclonal antibody, the EC50 of human CD276 protein is 1.961-2.243 ng/ml.
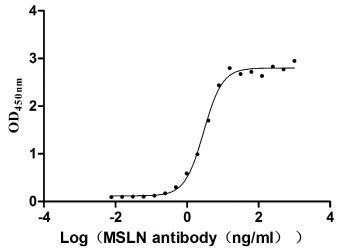
Measured by its binding ability in a functional ELISA. Immobilized MSLN at 2 μg/ml can bind Anti-MSLN rabbit monoclonal antibody, the EC50 of the MSLN protein is 2.657-3.177 ng/ml.
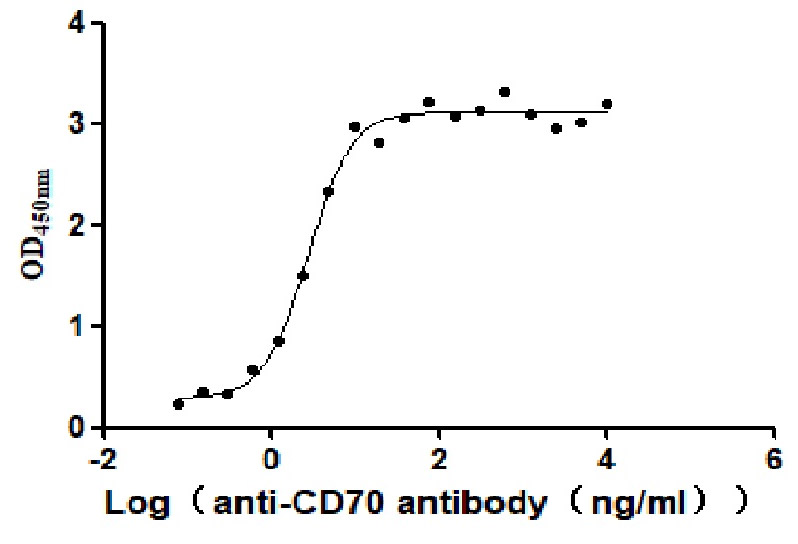
Measured by its binding ability in a functional ELISA. Immobilized Human CD70 at 2 μg/ml can bind Anti-CD70 antibody, the EC50 is 2.414-3.196 ng/mL.
● CUSABIO ADC Target Proteins
| Product Name |
Code |
Target |
Source |
Tag Info |
| Recombinant Human B-lymphocyte antigen CD19 (CD19), partial (Active) |
CSB-AP005061HU |
CD19 |
Mammalian cell |
C-terminal Fc-tagged |
| Recombinant Human B-cell receptor CD22 (CD22), partial (Active) |
CSB-MP004900HU |
CD22 |
Mammalian cell |
C-terminal 6xHis-tagged |
| Recombinant Human Programmed cell death 1 ligand 1 (CD274), partial (Active) |
CSB-MP878942HU1 |
CD274 |
Mammalian cell |
C-terminal hFc-tagged |
| Recombinant Human CD276 antigen (CD276), partial (Active) |
CSB-MP733578HU |
CD276 |
Mammalian cell |
C-terminal hFc-Myc-tagged |
| Recombinant Human Myeloid cell surface antigen CD33 (CD33), partial (Active) |
CSB-MP004925HU |
CD33 |
Mammalian cell |
C-terminal hFc-Myc-tagged |
| Recombinant Human Leukocyte surface antigen CD47 (CD47), partial (Active) |
CSB-AP005201HU |
CD47 |
Mammalian cell |
C-terminal 6xHis-tagged |
| Recombinant Human Leukocyte surface antigen CD47 (CD47), partial (Active) |
CSB-AP005211HU |
CD47 |
Mammalian cell |
C-terminal Fc-tagged |
| Recombinant Human Leukocyte surface antigen CD47 (CD47), partial (Active) |
CSB-MP004940HU |
CD47 |
Mammalian cell |
C-terminal hFc-tagged |
| Recombinant Human CD70 antigen (CD70), partial (Active) |
CSB-MP004954HU1 |
CD70 |
Mammalian cell |
N-terminal 10xHis-tagged |
| Recombinant Human HLA class II histocompatibility antigen gamma chain (CD74), partial (Active) |
CSB-MP004956HU1(F2) |
CD74 |
Mammalian cell |
N-terminal 10xHis-tagged |
| Recombinant Human Carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5) (E398K) (Active) |
CSB-MP005165HU |
CEACAM5 |
Mammalian cell |
C-terminal 10xHis-tagged |
| Recombinant Macaca fascicularis Claudin (CLDN18)-VLPs (Active) |
CSB-MP4304MOV |
CLDN18 |
Mammalian cell |
N-terminal 6xHis-tagged (This tag can be tested only under denaturing conditions) |
| Recombinant Mouse Claudin-18 (Cldn18)-VLPs (Active) |
CSB-MP005498MO(F3) |
Cldn18 |
Mammalian cell |
N-terminal 6xHis-tagged (This tag can be tested only under denaturing conditions) |
| Recombinant Human Claudin-6 (CLDN6)-VLPs (Active) |
CSB-MP005508HU(A4) |
CLDN6 |
Mammalian cell |
C-terminal 10xHis-tagged (This tag can be tested only under denaturing conditions) |
| Recombinant Human Claudin-6 (CLDN6)-VLPs, Fluorescent (Active) |
CSB-MP005508HU(A4)f4 |
CLDN6 |
Mammalian cell |
C-terminal GFP-tagged (This tag can be tested only under denaturing conditions) |
| Recombinant Human Delta-like protein 3 (DLL3), partial (Active) |
CSB-MP882142HU |
DLL3 |
Mammalian cell |
C-terminal 6xHis-tagged |
| Recombinant Macaca fascicularis Delta-like protein 3 (DLL3), partial (Active) |
CSB-MP3536MOV |
DLL3 |
Mammalian cell |
C-terminal 6xHis-tagged |
| Recombinant Human Epidermal growth factor receptor (EGFR), partial (Active) |
CSB-MP007479HU |
EGFR |
Mammalian cell |
N-terminal 10xHis-tagged and C-terminal Myc-tagged |
| Recombinant Human Receptor tyrosine-protein kinase erbB-3 (ERBB3), partial (Active) |
CSB-MP007765HU |
ERBB3 |
Mammalian cell |
C-terminal hFc-tagged |
| Recombinant Human G-protein coupled receptor family C group 5 member D (GPRC5D)-VLPs (Active) |
CSB-MP882153HU |
GPRC5D |
Mammalian cell |
C-terminal 10xHis-tagged (This tag can be tested only under denaturing conditions) |
| Recombinant Human Heat-stable enterotoxin receptor (GUCY2C), partial (Active) |
CSB-MP010053HU |
GUCY2C |
Mammalian cell |
N-terminal 10xHis-tagged |
| Recombinant Human Heat-stable enterotoxin receptor (GUCY2C), partial (Active) |
CSB-MP010053HUd9 |
GUCY2C |
Mammalian cell |
C-terminal hFc-tagged |
| Recombinant Human Hepatocyte growth factor receptor (MET), partial (Active) |
CSB-AP003691HU |
MET |
Mammalian cell |
C-terminal Fc-tagged |
| Recombinant Human Hepatocyte growth factor receptor (MET), partial (Active) |
CSB-MP013714HU |
MET |
Mammalian cell |
C-terminal hFc-tagged |
| Recombinant Human B-lymphocyte antigen CD20 (MS4A1)-VLPs (Active) |
CSB-MP015007HU |
MS4A1 |
Mammalian cell |
C-terminal 10xHis-tagged (This tag can be tested only under denaturing conditions) |
| Recombinant Dog B-lymphocyte antigen CD20 (MS4A1)-VLPs (Active) |
CSB-MP661636DO |
MS4A1 |
Mammalian cell |
C-terminal 10xHis-tagged (This tag can be tested only under denaturing conditions) |
| Recombinant Macaca fascicularis Membrane spanning 4-domains A1 (MS4A1)-VLPs (Active) |
CSB-MP4516MOV |
MS4A1 |
Mammalian cell |
C-terminal 10xHis-tagged (This tag can be tested only under denaturing conditions) |
| Recombinant Human Mesothelin (MSLN), partial (Active) |
CSB-MP015044HUc9 |
MSLN |
Mammalian cell |
N-terminal hFc-tagged |
| Recombinant Human Nectin-4 (NECTIN4), partial (Active) |
CSB-AP005571HU |
NECTIN4 |
Mammalian cell |
C-terminal 6xHis-tagged |
| Recombinant Human Nectin-4 (NECTIN4), partial (Active) |
CSB-MP822274HU |
NECTIN4 |
Mammalian cell |
C-terminal 10xHis-tagged |
| Recombinant Human Inactive tyrosine-protein kinase transmembrane receptor ROR1 (ROR1), partial (Active) |
CSB-MP020067HU1d7 |
ROR1 |
Mammalian cell |
C-terminal 10xHis-tagged |
| Recombinant Human Zinc transporter ZIP6 (SLC39A6), partial (Active) |
CSB-BP621669HU1 |
SLC39A6 |
Baculovirus |
C-terminal 6xHis-tagged |
| Recombinant Human Tumor-associated calcium signal transducer 2 (TACSTD2), partial (Active) |
CSB-MP023072HU1 |
TACSTD2 |
Mammalian cell |
C-terminal hFc-tagged |
| Recombinant Human Tumor-associated calcium signal transducer 2 (TACSTD2), partial (Active) |
CSB-MP023072HU2 |
TACSTD2 |
Mammalian cell |
C-terminal 10xHis-tagged |
| Recombinant Human Tumor-associated calcium signal transducer 2 (TACSTD2), partial (Active) |
CSB-MP023072HU1d7 |
TACSTD2 |
Mammalian cell |
C-terminal 10xHis-tagged |
| Recombinant Human Tumor necrosis factor receptor superfamily member 10B (TNFRSF10B), partial (Active) |
CSB-AP004921HU |
TNFRSF10B |
Mammalian cell |
C-terminal 6xHis-Fc-tagged |
| Recombinant Human Tumor necrosis factor receptor superfamily member 10B (TNFRSF10B), partial (Active) |
CSB-AP004931HU |
TNFRSF10B |
Mammalian cell |
C-terminal 6xHis-tagged |
| Recombinant Mouse Tumor necrosis factor receptor superfamily member 10B (Tnfrsf10b), partial (Active) |
CSB-AP005041MO |
Tnfrsf10b |
Mammalian cell |
C-terminal Fc-tagged |
| Recombinant Human Tumor necrosis factor receptor superfamily member 17 (TNFRSF17), partial (Active) |
CSB-MP023974HU1 |
Tnfrsf17 |
Mammalian cell |
C-terminal hFc-tagged |
| Recombinant Human Tumor necrosis factor receptor superfamily member 8 (TNFRSF8), partial (Active) |
CSB-MP023983HU1 |
TNFRSF8 |
Mammalian cell |
N-terminal 10xHis-tagged and C-terminal Myc-tagged |
| Recombinant Macaca fascicularis Trophoblast glycoprotein (TPBG), partial (Active) |
CSB-MP024093MOV |
TPBG |
Mammalian cell |
N-terminal 10xHis-tagged and C-terminal Myc-tagged |
| Recombinant Human Trophoblast glycoprotein (TPBG), partial (Active) |
CSB-MP024093HUb0 |
TPBG |
Mammalian cell |
N-terminal 10xHis-tagged |
References
[1] Abuhelwa Z, Alloghbi A, Nagasaka M. A comprehensive review on antibody-drug conjugates (ADCs) in the treatment landscape of non-small cell lung cancer (NSCLC)[J]. Cancer treatment reviews, 2022, 106.